Chromatography, chromatograph, and chromatogram: What are the (surprisingly unknown?) differences between these?
The words “chromatography,” “chromatograph,” and “chromatogram” come up often in chemical analysis.
These may sound similar, but did you know that they have different meanings?
This column will explain those differences.
“Chromatography” is a separation technique.
The separation of mixtures into their constituent pure substances is a vital part of chemical analysis.
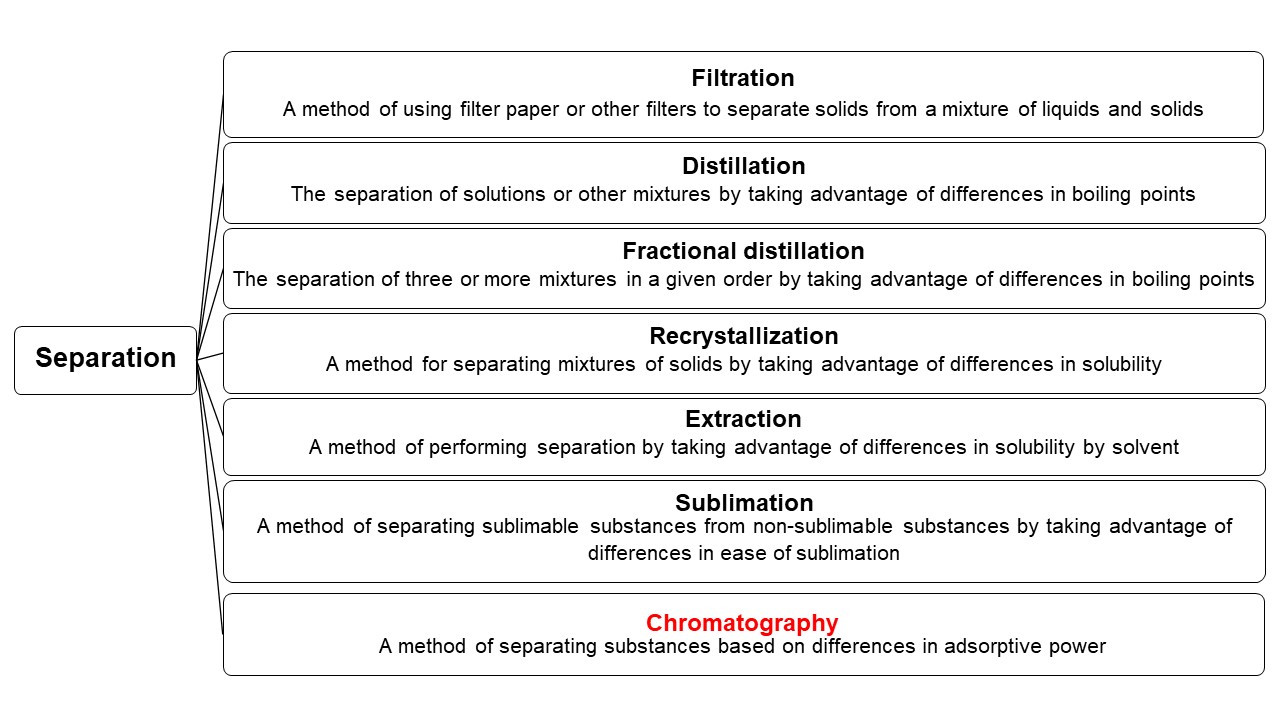
Chromatography is a general term encompassing methods for separating substances based on differences in adsorptive power. Other separation methods include filtration, which separates liquids from solids, and distillation, which uses the difference in boiling points of substances to perform separation.
Chromatography methods are broadly divided into liquid chromatography and gas chromatography, depending on whether the mobile phase is liquid or gas. Further subdivision is possible according to the type of the stationary phase and differences in techniques.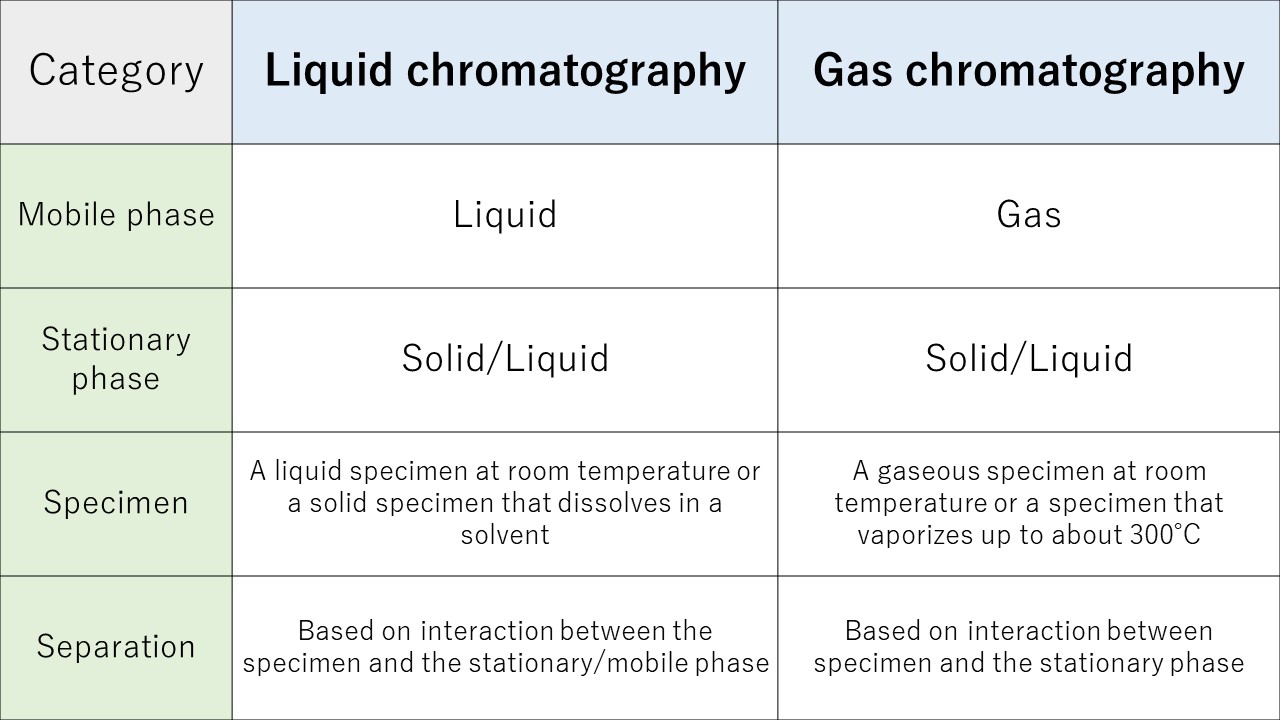
A “chromatograph” is an analytical instrument.
An analytical instrument that uses chromatography is called a chromatograph.
The combination of the instrument with a detector enables quantitative analysis of substances and is commonly used in many fields.
This is an essential analytical technology for quality control in the food industry, used in analysis of savory components, analysis of food additives, inspection of residual pesticides and toxic substances, and other areas.
In the pharmaceutical industry, the technology makes considerable contribution to drug development, particularly in analysis of active ingredients and identification of byproducts when ingested.
A “chromatogram” is an outcome of measurements.
A chromatogram is the result obtained when analysis is performed using a chromatograph (an analyzer).
The horizontal axis of the chromatogram expresses time and the vertical axis expresses signal value (intensity, absorbance, count, etc.).
A calibration curve is then used to convert the signal value to a concentration (amount) and to derive the concentration of the substance.
Summary
The above was an introduction to terms concerning chromatography.
- “Chromatography” is a separation technique.
- A “chromatograph” is an analytical instrument.
- A “chromatogram” is an outcome of measurements.
If you have any concerns about your water purifier, questions about water, requests for columns, or anything else, we welcome your inquiries here. We look forward to hearing from you!





